Pick a Topic:
Find info on a:

ClotCare complies with the HONcode standard for trustworthy health
information:
verify here.
 ClotCare is a member organization of the Coalition to Prevent Deep Vein Thrombosis. Click here to learn more about the Coalition to Prevent Deep Vein Thrombosis and DVT Awareness Month, which is held each March.
ClotCare is a member organization of the Coalition to Prevent Deep Vein Thrombosis. Click here to learn more about the Coalition to Prevent Deep Vein Thrombosis and DVT Awareness Month, which is held each March.
|
A Summary and Update on Combining Antiplatelet Agents and/or Oral Anticoagulants
February, 2014
Editor's Note from Henry I. Bussey, Pharm.D.: Below are two interesting and informative summaries on what is known currently about the risks, benefits, and optimal duration of combination therapy to prevent thrombosis. The first summary is from a recent newsletter of the Cardiology Practice and Research Network (PRN) of the American College of Clinical Pharmacy. The second summary (which follows the reference listing of the first summary), is an update to the first summary provided by Dr. Sarah Spinler (a member of the ClotCare Editorial Board) and one of her students, Chris Pham.
From the ACCP Cardiology PRN Newsletter
Managing Patients on Triple Antithrombotic Therapy
Erin Santiago PharmD, Kathleen Packard PharmD, MS, BCPS (AQ Card), Estella Davis PharmD, BCPS
Background
Approximately 5% of patients undergoing percutaneous coronary intervention (PCI) have an indication for anticoagulation in addition to dual antiplatelet therapy (DAPT), also known as “triple therapy”.1 Most have a history of atrial fibrillation (AF).2 Evidence for managing these patients is limited, especially involving the new oral anticoagulants and P2Y12 receptor antagonists.
Triple Therapy with Aspirin, Clopidogrel, and Warfarin
Triple therapy appears to be superior in preventing major adverse cardiac events (MACE) in PCI patients. Bleeding may be as much as 5-fold higher with triple therapy compared to DAPT.2-4 Bleeding risk appears to be highest after the initial acute coronary syndrome (ACS) event, decreases over time, and yet remains consistently higher with triple therapy compared to DAPT.3 This timing is significant because the greatest risk of stent thrombosis also occurs in the month after stent placement, thus requiring consideration of both bleeding and thrombotic risk.5
Triple Therapy with More Potent P2Y12 Receptor Antagonists
A recently published observational study in 377 patients receiving a drug eluting stent (DES) with an indication for triple therapy compared clopidogrel (n=356) and prasugrel (n=21).6 Prasugrel was associated with significantly more major and minor bleeding (29% versus 7%; adjusted HR 3.2; 95% CI 1.1-9.1; p=0.03) and a non-significant higher risk of MACE (9.5% versus 7.0%, HR 1.4, 95% CI 0.3-6.1, p=0.61) compared to clopidogrel. Based on this study, the lack of data with ticagrelor, and an increased risk of bleeding with the newer antiplatelet agents, only clopidogrel can be recommend for use in triple therapy.5,7
Triple Therapy with the New Oral Anticoagulants
When combined with DAPT in the setting of ACS, dabigatran, rivaroxaban, and apixaban have all been associated with increased bleeding.8-10 In the RE-DEEM trial (n=1,861; dabigatran 50 mg , 75 mg, 110 mg, or 150 mg BID versus placebo in addition to DAPT), a dose-dependent increase in bleeding events was observed.8 Furthermore, both the RE-LY trial and a recent meta-analysis have demonstrated an increased risk of MI with dabigatran in patients with AF.11-12 In the ATLAS ACS-TIMI 51 trial (n=15,526), rivaroxaban in combination with standard post-ACS antiplatelet therapy reduced death from any cause by 19% compared to placebo (p=0.04), but increased the risk of major bleeding (2.1% versus 0.6%, p<0.001) and intracranial hemorrhage (0.6% versus 0.2%, p=0.009).9 Rivaroxaban doses lower than approved AF dosing, 2.5 mg and 5 mg BID, were used in this study. The PIONEER trial will be moving forward with rivaroxaban 2.5 mg BID plus DAPT or rivaroxaban 15 mg daily plus a P2Y12 receptor antagonist compared to standard triple therapy.13 This prospective randomized trial in approximately 2,110 patients will hopefully shed light regarding the risk benefit ratio of 12 months of triple therapy with rivaroxaban in AF patients undergoing PCI.13 The APPRAISE-2 trial (n=7,392) for apixaban in ACS was terminated early due to the increased risk of TIMI major bleeding (1.3% vs. 0.5%, p=0.001) with no reduction in recurrent ischemic events compared to placebo.10
Management Strategies
While warfarin is superior to DAPT in the prevention of vascular events in patients with AF, DAPT in the first 12 months may be appropriate in some patients.14 The 2012 ACCP guidelines recommend triple therapy for one month after a bare-metal stent (BMS) and 3-6 months after a DES for patients with a CHADS2 score > 2 (Grade 2C).15 After triple therapy, the guidelines suggest to continue warfarin and a single antiplatelet up to 12 months. For patients with a CHADS2 score 0-1, DAPT alone is recommended for 12 months if a stent is placed (Grade 2C). After the first 12 months, the guidelines recommend continuing with warfarin monotherapy for all patients (Grade 2C).
In 2011, a North-American consensus statement was published providing more specific guidance for triple therapy patients, taking into account stent type, bleeding risk and thrombosis risk.5 The guidelines mention use of the HAS-BLED score, which has been recently shown to predict bleeding with moderate accuracy in triple therapy patients, as well as improved assessment of thrombotic risk with the CHA2DS2-VASc score.2,5
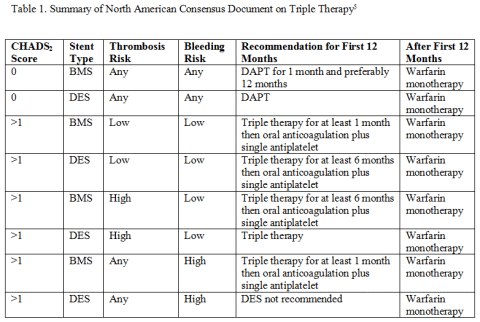
The only randomized trial assessing double versus triple antithrombotic therapy to date is the recently published “What is the Optimal antiplatElet and anticoagulant therapy in patients with oral anticoagulation and coronary StenTing” (WOEST) trial.16 This multicenter, randomized, open-label trial in 573 PCI patients assessed double therapy with warfarin plus clopidogrel or triple therapy including aspirin. The primary outcome of any bleeding within one year was significantly lower in double therapy patients compared to triple therapy (19.4% versus 44.4%, HR 0.36, 95% CI 0.26-0.50, p<0.0001). The rate of mortality was significantly higher for patients on triple therapy compared to double therapy (6.3% versus 2.5%, HR 0.39, 95% CI 0.16-0.93, p=0.027). While the study was not adequately powered to detect differences in stent thrombosis, there were no significant differences in the rate of thrombotic and thromboembolic events between the two groups.
In addition to drug selection, there are other opportunities for pharmacists to manage triple therapy patients. In the periprocedural setting, bivalirudin may be the preferred anticoagulant agent for these patients due to lower risk of bleeding.7 The 2012 ACCF/AHA UA/NSTEMI guidelines give a class IIb recommendation for targeting warfarin to a lower INR (2.0-2.5) in patients requiring triple therapy.17 This reduced therapeutic range with tight control may be difficult to achieve in some patients.5 A thorough assessment of patient adherence should be made prior to PCI as premature discontinuation of DAPT, especially in the first few months, is associated with a 5-36 fold risk increase in stent thrombosis.5 Low dose aspirin is recommended due to equal benefit as higher doses with fewer GI bleeds.7 NSAID use should be avoided and gastric protection with a proton pump inhibitor should be considered in all patients, taking into account drug-drug interactions.4,5,7 In the future, pharmacists may also play a role in the selection and management of patients receiving left atrial appendage closure devices (WATCHMAN® device) which may obviate the need for long term anticoagulation.18
Conclusion
Pharmacists can play a role in the management of triple therapy through an assessment of thromboembolic risk, risk of bleeding, and adherence. Pharmacists should be vigilant to assess appropriateness of DAPT versus triple therapy and to recommend an appropriate duration of pharmacotherapy post-PCI. New P2Y12 receptor antagonists and oral anticoagulants lack specific recommendations for their place in triple therapy. Due to increased risk of bleeding, further randomized, controlled trials will be necessary to assess their place in therapy.
Editor’s Note: See updated comments below reference listing from Mr. Pham and Dr. Spinler.
References
- Rubboli A, Colletta M, Valencia J, et al. Periprocedural management and in-hospital outcome of patients with indication for oral anticoagulation undergoing coronary artery stenting. J Interven Cardiol 2009;22:390-7.
- Smith JG, Wieloch M, Koul S et al. Triple antithrombotic therapy following an acute coronary syndrome: prevalence, outcomes and prognostic utility of the HAS-BLED score. EuroIntervention 2012;8(6):672-8.
- Lamberts M, Olesen JB, Ruwald MH, et al. Bleeding after initiation of multiple antithrombotic drugs, including triple therapy, in atrial fibrillation patients following myocardial infarction and coronary intervention: a nationwide cohort study. Circulation 2012;126:1185-93.
- Menozzi M, Rubboli A, Manari A et al. Triple antithrombotic therapy in patients with atrial fibrillation undergoing coronary artery stenting: hovering among bleeding risk, thromboembolic events, and stent thrombosis. Thromb J 2012;10:1-11.
- Faxon DP, Eikelboom JW, Berger PB, et al. Consensus document: antithrombotic therapy in patients with atrial fibrillation undergoing coronary stenting. Thromb Haemostasis 2011;106:571-84.
- Sarafoff N, Martischnig A, Wealer J, et al. Triple therapy with aspirin, prasugrel, and vitamin K antagonists in patients with drug eluting stents implantation and an indication for oral anticoagulation. J Am Coll Cardiol 2013; February epub ahead of print.
- Faxon DP. How to manage antiplatelet therapy for stenting in a patient requiring oral anticoagulants. Curr Treat Options in Cardiovasc Med 2013;15:11-20.
- Oldgren J, Budaj A, Granger CB, et al; RE-DEEM Investigators. Dabigatran vs. placebo in patients with acute coronary syndrome on dual antiplatelet therapy: a randomized, double-blind, phase II trial. Eur Heart J 2011; 32: 2781-9.
- Mega JL, Braunwald E, Wiviott SD, et al. ATLAS ACS 2-TIMI 51 Investigators. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med 2012; 366: 9-19.
- Alexander JH, Lopes RD, James S, et al. APPRIASE-2 Investigators. Apixaban with antiplatelet therapy after acute coronary syndrome. N Engl J Med 2011; 365: 699-708.
- Artang R, Rome E, Vidaillet, et al. Dabigatran and myocardial infarction, drug or class effect. Meta-analysis of randomized trials with oral direct thrombin inhibitors. J Am Coll Cardiol 2012;59(13):E571 (Abstract).
- Dans AL, Connolly SJ, Wallentin L, et al. Concomitant use of antiplatelet therapy with dabigatran or warfarin in the randomized evaluation of long-term anticoagulation therapy (RE-LY) trial. Circulation 2013; 127:634-40.
- ClinicalTrials.gov website. Accessed May 21, 2013. Available at: http://www.clinicaltrials.gov/ct2/show/NCT01830543.
- Connolly SJ, Pogue J, Hart RG, et al. ACTIVE Investigators, Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med 2009;360:2066-78.
- You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e531S-e575S.
- Dewilde WJM, Oirbans T, Verheugt FWA, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomized, controlled trial. Lancet 2013;381(9872):1107-15.
- Jneid H, Anderson JL, Wright RS, et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/Non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update). Circulation 2012;126:875-910.
- Holmes DR. Randomized trial of LAA closure vs warfarin for stroke/ thromboembolic prevention in patients with non-valvular atrial fibrillation (PREVAIL). Am Coll Cardiol Scientific Sessions March 2013 San Francisco, California.
Update to 2013 Guidelines for the Management of ST-Elevation Myocardial Infarction (STEMI)
An Update from Dr. Spinler and Mr. Pham
Christopher Pham, Pharm. D. Candidate and Sarah A. Spinler, Pharm.D., Philadelphia College of Pharmacy, University of the Sciences in Philadelphia
The 2013 American College of Cardiology Foundation/American Heart Association guidelines recommend triple antithrombotic therapy following percutaneous coronary intervention (PCI) stent placement in patients with atrial fibrillation and a CHADS2 score ≥ 2 or those with another need of for oral anticoagulation such as a mechanical heart valve, venous thromboembolism, or hypercoagulable disorder (Class 1 recommendations). Triple therapy is also recommended for patients with asymptomatic left ventricular thrombus, but this is a Class IIa recommendation. Other patients where triple antithrombotic therapy can be considered are patients with STEMI and anterior apical akinesis or dyskinesis (Class IIb recommendations) in whom it is recommended to limit the duration of vitamin K antagonist (VKA) therapy to 3 months.1
The duration of triple therapy should be minimized to limit the risk of bleeding (Class 1 recommendation), and is dependent on stent type. No specific recommendation is given for the duration of therapy for a bare metal stent (BMS) or drug eluting stent (DES), but the guidelines suggest avoiding a DES in patients requiring anticoagulation. The guidelines also recommend considering a lower international normalized ratio (INR) target (2.0-2.5) in patients who are receiving triple therapy (Class IIb recommendation) but acknowledge that there are no data that affirm this target INR range. For patients with a CHADS2 score of 0 or 1, dual antiplatelet therapy (DAPT) alone for 12 months is recommended. 81 mg is the preferred maintenance dose of aspirin in patients all patients (Class IIa recommendation).1
Update from a recent meta-analysis
A recent meta-analysis of all phase II and III studies of the new oral anticoagulants in acute coronary syndrome examined the effect of adding a new oral anticoagulant to DAPT. Adding one of the new oral anticoagulants to DAPT decreased the risk major adverse cardiac events (MACE) from 4.3% to 3.6% (HR 0.87; 95% CI 0.80-0.95) and significantly increased the risk of clinically significant bleeding from 3.1% to 7.1% (HR 2.34; 95% CI 2.06-2.66). The number needed to treat for six months to prevent one event was 187 while the number needed to treat to harm an additional patient was 24. This study showed only a small benefit to adding a new oral anticoagulant to DAPT but a substantial increase in bleeding risk. Based on this study, the new oral anticoagulants should not be recommended for use in triple therapy.2
References
- American College of Emergency Physicians, Society for Cardiovascular Angiography and Interventions, O'Gara PT, Kushner FG, Ascheim DD, Casey DE,Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Brindis RG, Creager MA, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Kushner FG, Ohman EM, Stevenson WG, Yancy CW. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American college of cardiology Foundation/American heart association task force on practice guidelines. J Am Coll Cardiol 2013;61(4):e78-140.
- Oldgren J, Wallentin L, Alexander JH, James S, Jonelid B, Steg G, Sundstrom J. New oral anticoagulants in addition to single or dual antiplatelet therapy after an acute coronary syndrome: A systematic review and meta-analysis. Eur Heart J. 2013 Jun;34(22):1670-80.
|
ClotCare is a 501(c)(3) non-profit organization generously supported by your tax-deductible donations and grants from our industry supporters.
New Postings:
Click here to view full list of new postings
|